2020 Hepatitis C Testing, Treatment and Research News
(click news item title to open/close)
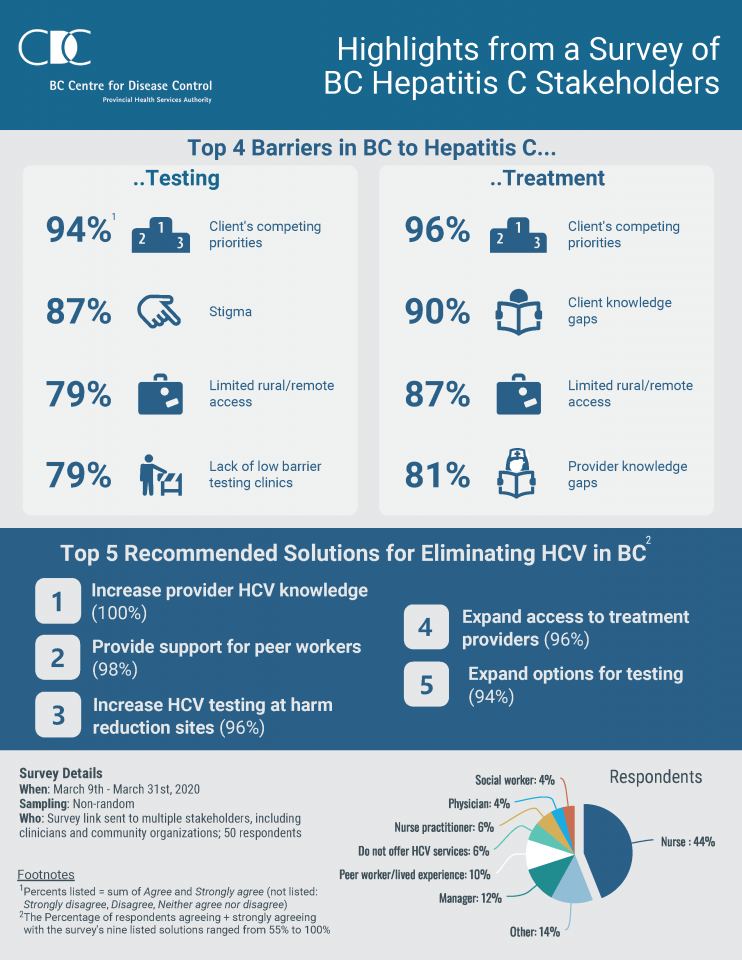
British Columbia BC:HCV Stakeholder Survey Highlights
In March 2020, BCCDC conducted the Hepatitis C Stakeholders Survey to gather input from Hepatitis C (HCV) stakeholders such as service and treatment providers on HCV education, prevention, support, testing and treatment services and programs in BC. The results of the survey will be used to inform coordination of HCV-related services and future HCV elimination efforts.
British Columbia - NON-URGENT HCV Testing Re-Started
June 15, 2020

View/Download full details here: BC CDC | BC Ministry of Health
Routine/non-urgent HCV testing at the BCCDC Public Health Laboratory (PHL) has been restarted.
British Columbia - Temporary Suspension of NON-URGENT HCV Testing
April 9, 2020

View/Download full details here: BC CDC | BC Ministry of Health
Routine/non-urgent HCV testing at the BCCDC Public Health Laboratory (PHL) is temporarily suspended to redirect resources to support COVID-19 testing. This includes all routine HCV screening and testing related to HCV treatment. The BCCDC PHL performs more than 95% of all BC’s HCV testing.
When HCV testing resumes, health care providers will be notified. People can sign up for HCV testing reminders through SmartSexResource.com, or sign up to the STI Updates blog to receive a notification when HCV testing resumes.
2019 Hepatitis C Treatment and Research News
Blueprint to inform hepatitis C elimination efforts in Canada
May 2019
The Canadian Network on Hepatitis C (CanHepC) released their blueprint to inform Canada's HCV elimination efforts.
The Blueprint to inform hepatitis C elimination efforts in Canada was developed by CanHepC, through a consultative process, to define what needs to be done to achieve hepatitis C virus (HCV) elimination in Canada. It’s a menu of options to help provinces and territories develop their own HCV action plans to ensure we meet the World Health Organisation HCV elimination targets.
Visit CanHepC to download the document.
2018 Hepatitis C Treatment and Research News
CASL Updated HCV Treatment Guidelines
June 4, 2018
The Canadian Association for the Study of the Liver has updated their hepatitis C management guidelines. Click below to view.
The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver
VOSEVI on BC, Ontario and Quebec drug plans
April 11, 2018
VOSEVI™ (sofosbuvir, velpatasvir/voxilaprevir) is now available on BC, Ontario and Quebec drug plans (formularies). The treatment involves taking one pill (with food) per day for 12 weeks.
VOSEVI™ is for patients with chronic hepatitis C (with or without cirrhosis), who have
-
- genotype 1,2,3,4,5, or 6 infection AND previously treated with an NS5A inhibitor containing regimen
- genotype 1,2,3, or 4 infection AND previously treated with a sofosbuvir containing regimen without NS5A inhibitor
For more details visit CATIE
For contraindications and symptoms see Product monograph
All hepatitis C patients now eligible for treatment in BC and Ontario
March 13 2018
British Columbia
Today it was announced that all residents of British Columbia are now eligible for chronic hepatitis C treatment. For more details, see the BC Ministry of Health News Release.
Ontario
The Ontario ministry of health expanded hepatitis C treatment to all people living with hepatitis C.
For more details, see the CBC News report.
2017 Hepatitis C Treatment and Research News
Two new pan-genotypic hepatitis C drugs approved in Canada
August 17 2017
Pan-genotypic drugs MAVIRET (AbbVie) and VOSEVI (Gilead) have been approved for hepatitis C treatment in Canada.
MAVIRET: 8-weeks, three tablets (once daily), new to treatment
MAVIRET (glecaprevir/pibrentasvir) is approved for those new to treatment, including:
-
-
- genotypes 1, 2, 3, 4, 5 and 6
- with or without cirrhosis
- with or without chronic kidney disease (all stages)
-
MAVIRET is also approved for those with genotype 1 and previously treated with certain direct-acting antivirals (DAAs). Selected warnings (based on U.S. "MAVYRET" patient information; Canadian monograph not yet available online) include:
-
-
- potential for hepatitis B re-activation
- should not be taken with certain liver problems
-
For more information, see: AbbVie's press release.
VOSEVI: 12-weeks, single tablet (once daily), previously treated
VOSSEVI (sofosbuvir/velpatasvir/voxilaprevir) is approved for those who have been previously treated with:
-
-
- an NS5A inhibitor-containing treatment (and genotype 1, 2, 3, 4, 5 or 6), or
- sofosbuvir-containing treatments without an NS5A inhbitor (and genotype 1, 2, 3, or 4)
-
Selected warnings include:
-
-
- Serious symptomatic bradycardia when co-administered with Amiodarone
- potential for hepatitis B re-activation
- in trials: 2% had one serious side-effect
- co-administration with digoxin may increase the concentration of digoxin
-
For more information, see:
More work needed to improve engagement in hepatitis C care
July 31 2017
There is much excitement about the new, highly curative treatments for HCV that can reduce illness and death associated with this virus. However, the potential impact of these new drugs will be blunted if people affected by HCV are not encouraged to move along the HCV Cascade of Care.
BC data shows that many people affected by HCV either do not get into or fall out of care. For example, many people who are diagnosed as antibody positive do not receive a follow-up test to confirm that the HCV virus is still in their body. Those less likely to be RNA tested include males and people born before 1975. Also, based on 2012 data, only about one-third of those living with HCV receive liver-related care or monitoring in a given year[7]. People affected by HCV need support and encouragement to receive the care and treatment they need. [See more details and references]

Patient resources
Order print resources
About the BC Hepatitis Testers Cohort
July 28 2017 is World Hepatitis Day
Knowledge generation to save lives from Hepatitis C!
July 28 2017
The British Columbia Hepatitis Testers Cohort (BC-HTC) is one of the most comprehensive population based cohorts in the world. It includes all individuals (~ 1.7 million) tested for HCV, or HIV in British Columbia, or reported to public health as a confirmed case of HCV, HBV, HIV/AIDS, since 1990. It is linked with demographics, medical visits, hospitalizations, prescription drugs, cancers and deaths to create a longitudinal medical history.
The overall purpose of the BC-HTC is to monitor disease burden related to hepatitis and associated infections and social conditions, evaluate impact of interventions, and monitor hepatitis program progress to inform policy and programming in British Columbia and Canada.
Important findings
Using BC-HTC's anonymized data, researchers have recently published some high profile papers, including:
-
-
- the largest study on hepatitis C reinfection in the World in the Lancet Hepatology & Gastroenterology,
- papers on the impact of liver cancer treatment as well as delays in hepatitis diagnoses (Journal of Hepatology) on patient outcomes, and
- the first study presenting the entire population cascade of care.
-
Showcased at AASLD
Some of BC-HTC’s work has been showcased at the American Association of Study Liver Diseases’ The Liver Meeting’s Hepatitis Debrief, which summarises ground breaking research at the conference.
More information
Details on the BC-HTC cohort, including publications and presentations, are available at: http://bchtc.med.ubc.ca/.
CIHR Hepatitis Profile
To see what other Canadian researchers are doing, see CIHR's profile on Canadian researchers working together to eliminate hepatitis C.
Screening Recommendations Questioned
May 8 2017
Canadian hepatitis C experts have suggested the Canadian Taskforce HCV screening recommendations are out of date and recommend updating (Hepatitis C testing in Canada: Don't leave baby boomers behind, CMAJ May 4 2017).
Canadian Taskforce on Preventive Health Care Releases Screening Recommendations
April 24 2017
The Canadian Task Force on Preventive Health Care published its recommendations on hepatitis C screening for adults today (CMAJ 2017 April 24).
It does not recommend population-based screening for low-risk, asymptomatic adults. However, exceptions include pregnant women or those at elevated risk of HCV, including those (page E596):
-
-
- with current or history of injection drug use
- who have been incarcerated
- who were born, travelled or resided in HCV-endemic countries
- who have received health care where there is a lack of universal precautions
- who have received blood transfusions, blood products or organ transplant before 1992 in Canada
- on hemodialysis
- who have had needle-stick injuries
- who have engaged in other risks sometimes associated with HCV exposure, such as high-risk sexual behaviours, homelessness, intranasal and inhalation drug use, tattooing, body piercing or sharing sharp instruments or personal hygiene materials with someone who is HCV positive
- with clinical clues suspicious for HCV infection and above risk factors
-
Their recommendation included the following considerations:
-
-
- “the anticipated increase in harm resulting from diagnosing and treating individuals who screen positive but would have never developed HCV-related disease during their lifetime;
- false positives and false negatives, which could lead to unnecessary anxiety and/or false reassurance;
- the potential for screening to increase inequity, given that among those who do not meet current eligibility criteria (e.g., specific comorbidities), only wealthier individuals or those with private insurance would obtain earlier access to treatment not currently funded by government;*
- the unknown magnitude of benefit of treatment on reducing risk of transmission; and
- the very large impact that screening and treatment would have on health care budgets, and associated opportunity costs (i.e., the limit this would place on the ability to provide other health care interventions that would have to be forgone for lack of funds, despite being supported by better evidence)” (page E598)
-
*Note: New price negotiations by the pan-Canadian Pharmaceutical Alliance has initiated changes to fibrosis eligibility requirements (item 3 above). For example, starting in 2018-2019, all people with HCV will be eligible for drug coverage (see news article below).
Hepatitis C Drug Coverage to be Expanded
February 2017
The pan-Canadian Pharmaceutical Alliance (pCPA) has negotiated price adjustments that will result in expanded drug coverage to those affected by hepatitis C.
The pCPA negotiated with Gilead Sciences Canada, Merck Canada, and Bristol-Myers Squibb Canada for reduced costs on the following drugs:
-
-
- Daklinza (daclatasvir)
- Epclusa (sofosbuvir/velpatasvir)
- Harvoni (ledipasvir/sofosbuvir)
- Sovaldi (sofosbuvir)
- Sunvepra (asunaprevir)
- Zepatier (elbasvir/grazoprevir)
-
For British Columbia this means that starting March 21 2017, physicians can apply for new drugs. Then, starting 2018-2019, BC PharmaCare will provide drug coverage for people in BC living with chronic hepatitis C, regardless of the extent of liver damage.
Stay tuned for more information of hepatitis C drug coverage across Canada.
For more information, visit: BC Government News
2016 Hepatitis C Treatment News
EASL updated hepatitis C treatment recommendations
September 2016
The European Association for the Study of the Liver updated their hepatitis C treatment recommendations in September.
[EASL Summary of Recommendations]
Pan-genotypic hepatitis C anti-viral medication, Epclusa, approved in Canada
Jul 14, 2016
Health Canada has issued a Notice of Compliance to Gilead Sciences Canada, Inc. (Gilead Canada) for EPCLUSA (sofosbuvir 400 mg/velpatasvir 100 mg). EPCLUSA is a once-daily, pan-genotypic single tablet 12 week regimen for the treatment of adults with genotype 1-6 chronic hepatitis C virus (HCV) infection for use in patients without cirrhosis or with compensated cirrhosis, and in combination with ribavirin for patients with decompensated cirrhosis. It is the first single tablet regimen without the need for ribavirin approved for the treatment of patients with HCV genotype 2 and 3.
For more information, see:
Gilead Epclusa press release Jul 14, 2016
2016 Updated Canadian HIV/Hepatitis C Adult Guidelines for Management and Treatment
Jul 7, 2016
The standing working group convened by The Canadian Institute of Health Research HIV Trials Network to review recently published HCV antiviral data has updated Canadian HIV-HCV Coinfection Guidelines. Recommendations include that all coinfected individuals should be assessed for interferon-free, Direct Acting Antiviral HCV therapy.
For more information, see:
2016 Updated Canadian HIV/Hepatitis C Adult Guidelines for Management and Treatment
Updated: AASLD HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C
Jul 7, 2016
AASLD updated the HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. The update reflects several important developments, including the recent approval of sofosbuvir/velpatasvir, together with new information regarding the use of testing for HCV resistance associated variants.
For more information, see:
AASLD Update HCV Guidance Jul 6, 2016
All-oral pan-genotypic treatment for hepatitis C approved in U.S.
June 29, 2016
The U.S. Food and Drug Administration has approved Epclusa (sofosbuvir and velpatasvir) to treat those with hepatitis C (HCV) genotypes 1-6, with or without cirrhosis (Epclusa + Ribavirin for those with decompensated cirrhosis). In clinical trials, cure rates were:
-
-
- 98% for 3 clinical trials (1,015 of 1,035 patients cleared the virus)
- 94% for those with decompensated cirrhosis (Epclusa + Ribavirin for 12 weeks)
- 83% for those with decompensated cirrhosis (Epclusa alone for 12 weeks)
-
For more information, see:
Gilead Epclusa Press Release (June 28, 2016)
FDA Epclusa News Release (June 28, 2016)
New Hepatitis C Care and Treatment Guidelines from WHO
April 15, 2016
The World Health Organization (WHO) released "Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection" (April 2016).
The new guidelines include recommendations about screening, prevention, care and treatment. It also presents global prevalence estimates, the natural history of hepatitis C and summaries of treatment studies.
For the PDF report see WHO: Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection.
Health Canada Safety Review for Simeprevir
February 17, 2016
Health Canada has reported receiving 11 Canadian reports of severe liver problems, and two deaths, that MAY be associated with simeprevir (Galexos). A confirmed link between simeprevir and liver problems has not been made due to limited information, but the manufacturer recommends health care providers:
-
-
- Check for liver function before and during treatment
- Should not use simeprevir with patients that have moderate or severe liver damage
-
For more details see Health Canada’s Summary Safety Review for simeprevir.
Zepatier approved by Health Canada
January 25, 2016
Health Canada recently approved Zepatier (elbasvir + grazoprevir). This all-oral, interferon-free treatment is for people over the age of 18 years living with hepatitis C genotype 1, 3, or 4 and has overall cure rates of 92-97%.
Depending on a person’s genotype, treatment history, liver health (e.g., presence of cirrhosis), treatment with Zepatier can range from 8 to 16 weeks and it may be given:
-
-
- without ribavirin
- with ribavirin, or
- with sofosbuvir
-
Very common side-effects (occurring in more than 10% of those treated) include headache and feeling tired. See patient information page for a detailed list of common side-effects (occurring in 1 to 10% of those treated).
Click here for manufacturer’s patient information page.
Click here for more manufacturer’s product monograph.
2015 Treatment News
Daclatasvir/Sofosbuvir/Ribavirin - Cure rates as high as 89% for GT-3 Cirrhotic
December 2, 2015
Results from the Phase 3 Ally-3+ trial results were presented at AASLD in mid-November. For patients with cirrhosis who were treated with daclatasvir (Daklinza), sofosbuvir and ribavirin for 16 weeks, SVR rates were 89% (83% for those treated for 12 weeks). SVR rates were 100% for those with F3 fibrosis scores (12 or 16 weeks of treatment).
Common side-effects included insomnia (30%), fatigue (26%) and headache (24%)*.
Daclatasvir was approved by Health Canada August 13, 2015 for the treatment of hepatitis C.
For more detailed information, see:
Gilead U.S. application for pangenotypic HCV treatment
October 28, 2015
Gilead announced it has submitted a drug application to the U.S. Food and Drug Administration for a hepatitis C treatment regime for six genotypes of hepatitis C.
The new treatment:
-
-
- is all oral
- contains sofosbuvir and velpatasvir
- is for patients with HCV genotypes 1-6
- is expected to be 12 weeks in duration
- can be for patients with decompensated cirrhosis (with ribavirin)
-
Harvoni Coverage in Canada Update
August 27, 2015
Harvoni, an highly effective and well-tolerated therapy for the treatment of hepatitis C, is now listed on almost all provincial/territorial formularies:
-
-
- British Columbia [see BC Formulary]
- Alberta [see Online drug benefit list]
- Saskatchewan [Saskatchewan Formulary]
- Manitoba [see Man. Health Bull 82 (pg 3)]
- Ontario [see Ont. MOH EAP Mar 1/15 update]
- Quebec [List of Medications]
- New Brunswick [see N.B. Formulary]
- Newfoundland and Labrador [see Special Authorization Request Form]
- Nova Scotia [N.S. Formulary: PDF Online]
- Yukon [Y.T. Formulary]
- Northwest Territories and Nunavut list according to the Non-Insured Health Benefits [see NIHB Formulary]
-
Getting treatment can take time as several steps are involved (e.g., knowing one's hepatitis C genotype, having appointments with providers and/or specialists, having an assessment for the extent of liver damage).
For table summaries of hepatitis C treatment recommendations by AASLD (September 2017) click here.
Daclatasvir combination with Sovaldi got approved by Health Canada today.
This now provides a good combination of interferon free treatment for genotype 3.
July 28, 2015
BC Pharmacare now covers HOLKIRA PAK (ombitasvir, paritaprevir, ritonavir and dasabuvir) for hepatitis C treatment (genotype 1)
The British Columbia government announced today that HOLKIRA PAK (ombitasvir, paritaprevir, ritonavir and dasabuvir) will be covered by BC Pharmacare. People with hepatitis C genotype 1 can now apply for coverage. Cure rates are reported to be over 90%.
For more information, see
July 25, 2015
FDA approves TECHNIVIE (ombitasvir, paritaprevir, and ritonavir tablets) for the treatment of patients with genotype 4 chronic hepatitis C
The U.S. Food and Drug Administration (FDA) approved TECHNIVIE (ombitasvir, paritaprevir, and ritonavir tablets) in combination with ribavirin (RBV) for the treatment of adults with genotype 4 (GT4) chronic hepatitis C virus (HCV) infection who do not have cirrhosis. TECHNIVIE is the first and only all-oral, interferon-free, direct-acting antiviral treatment approved in the U.S. for adult patients with historically difficult to treat GT4 chronic HCV infection.
This treatment for GT4 has reported cure rates of 100% at 12 weeks post-treatment in patients without cirrhosis who took TECHNIVIE with ribavirin (RBV) for 12 weeks.
For more detailed information, see manufacturer information:
July 24, 2015
FDA approves Daklinza (daclatasvir) for the treatment of patients with hepatitis C genotype 3
Daklinza, in combination with sofosbuvir, is the first 12-week, all-oral therapy that offers SVR12 for most genotype 3 patients. Hepatitis C genotype 3 is one of the most difficult-to-treat genotypes. This is the first approval of Daklinza in the United States (See Bristol-Myers Squibb Company press release).
Reported SVR12 for the Daklinza plus sofosbuvir regimen was 90% for treatment-naïve and 86% for treatment-experienced chronic HCV genotype 3 patients. SVR12 rates were higher (96%) in genotype 3 patients without cirrhosis, regardless of treatment history. In the more difficult-to-treat patients with cirrhosis, SVR12 rates were reduced (63%). These SVR12 rates were achieved with 12 weeks of therapy without the use of ribavirin.
For more detailed information, see manufacturer information:
July 24, 2015
Holkira Pak is now available in Quebec
In addition to Prince Edward Island and Ontario, Holkira Pak (ombitasvir/paritaprevir/ritonavir tablets and dasabuvir tablets) is now available in Quebec (See Régie de l'assurance maladie du Québec).
This interferon-free treatment for hepatitis C genotype 1 has reported cure rates of over 95%. Duration of treatment ranges from 12 to 24 weeks.
To compare Holkira Pak with other hepatitis C genotype 1 treatments, see CASL Recommended HCV Treatment Genotype 1.
For a summary of Holkira Pak contents and use, see CATIE Holkira Pak Fact Sheet.
For more detailed information, see manufacturer information for:
June 30, 2015
Holkira Pak is now available in Ontario
In addition to Prince Edward Island, Holkira Pak (ombitasvir/paritaprevir/ritonavir tablets and dasabuvir tablets) is now available in Ontario (See Ontario Exceptional Access Program (EAP)).
This interferon-free treatment for hepatitis C genotype 1 has reported cure rates of over 95%. Duration of treatment ranges from 12 to 24 weeks.
To compare Holkira Pak with other hepatitis C genotype 1 treatments, see CASL Recommended HCV Treatment Genotype 1.
For a summary of Holkira Pak contents and use, see CATIE Holkira Pak Fact Sheet.
For more detailed information, see manufacturer information for:
April 27, 2015
Bristol-Myers Squibb announces acceptance of new drug application for investigational daclatasvir for FDA review for the treatment of hepatitis C genotype 3
On March 12, 2015 Bristol-Myers Squibb (BMS) announced that the resubmitted new drug application for daclatasvir, has been accepted for review by the U.S. Food and Drug Administration (FDA) for use in combination with sofosbuvir for the treatment of chronic hepatitis C genotype 3. [see BMS news]
April 27, 2015
Grazoprevir / Elbasvir highly effective in previously untreated hepatitis C
On friday April 24, 2015 at the Interntaional Liver Congress in Vienna, Austria, results of the C-EDGE trial were presented that showed a 12-week course of the combination of grazoprevir and elbasvir cured 95% of previously untreated people with genotypes 1, 4 or 6 hepatitis C virus. [see: New and Experimental Hepatitis C Treatment]
EASL Recommendations on Treatment of Hepatitis C 2015
April 27, 2015
The European Association for the Study of the Liver (EASL) released recommendations on Treatment of Hepatitis C at the International Liver Congress. These recommendations outline optimal management for patients with acute and chronic HCV infections.
Update on Harvoni availability in Canada
April 22, 2015
Harvoni, an highly effective and well-tolerated drug with cure rates of over 90%, is now available in the following provinces/territories*:
- British Columbia [see B.C. Gov't Newsroom Mar 23/15 or BC Formulary]
- Alberta [see Summary of Changes Apr 1/15 or Online drug benefit list]
- Saskatchewan [Saskatchewan Formulary]
- Manitoba [see Man. Health Bull 82 (pg 3)]
- Ontario [see Ont. MOH EAP Mar 1/15 update]
- New Brunswick [see N.B. Formulary Update Mar 23/15 or N.B. Formulary]
- Nova Scotia [N.S. Formulary: PDF Online]
- Yukon [Y.T. Formulary]
Quebec is not formally part of the pCPA discussions, but negotiations are in progress. The following territories will list according to the Non-Insured Health Benefits (NIHB) formulary (to be updated soon)**:
- Northwest Territories
- Nunavut
Getting treatment can take time as several steps are involved (e.g., knowing one's hepatitis C genotype, having appointments with providers and/or specialists, assessment for extent of liver damage).
Negotiations with the drug company that manufactures Harvoni were done through the Pan-Canadian Pharmaceutical Alliance (pCPA).
*Listing criteria not yet available online for all provinces/territories
**Source Canadian Liver Foundation (CLF), March 25, 2015, Personal Communication.
Also see CLF News Release.
WARNING: Harvoni or Sovaldi + DAA NOT to be combined with amiodarone
March 30, 2015
HEALTH WARNING: Very serious slowing of the heart rate "can occur when the antiarrhythmic drug amiodarone is taken together with either the hepatitis C drug Harvoni (ledipasvir/sofosbuvir) or with Sovaldi (sofosbuvir) taken in combination with another direct acting antiviral for the treatment of hepatitis C infection" (U.S. Food and Drug Administration safety alert, March 24, 2015.) [click on FDA reference for more details]
Harvoni availability across much of Canada!*
March 25, 2015
Across much of Canada, Harvoni (ledipasvir/sofosbuvir) is, or will soon be, available for the treatment of people with :
- hepatitis C genotype 1
- specific treatment histories, and
- a minimum level of liver damage (e.g., fibrosis stage 2 or higher; may vary by province/territory)
Harvoni is a highly effective and well-tolerated drug with cure rates of over 90% and treatment durations of up to 24 weeks. Negotiations with the drug company that manufactures Harvoni were done through the Pan-Canadian Pharmaceutical Alliance (pCPA).
However, the public needs to be aware that getting treatment can take time as several steps are involved (e.g., knowing one's hepatitis C genotype, having appointments with providers and/or specialists, assessment for extent of liver damage).
Harvoni availability dates by Province/Territory (note: listing criteria not yet available online for all provinces/territories)
- British Columbia Mar 24/2015 [see B.C. Gov't Newsroom Mar 23/15]
- Manitoba Apr 20/2015 [see Man. Health Bull 82 (pg 3)]
- Ontario Mar 24/2015 [see Ont. MOH EAP Mar 1/15 update]
- New Brunswick Mar 23/2015 [see N.B. Formulary Mar 23/15]
- Yukon Mar 10/2015 [see Y.T. Formulary and search “Harvoni”]
The following territories will list according to the Non-Insured Health Benefits (NIHB) formulary (to be updated soon):
- Northwest Territories
- Nunavut
The following provinces are part of the pCPA and discussions, and negotiations are in progress. Decisions are expected soon in most cases.
- Alberta
- Saskatchewan
- Nova Scotia
- Newfoundland
- Prince Edward Island
Quebec is not formally part of the pCPA discussions, but negotiations are in progress.
*Source Canadian Liver Foundation (CLF), March 25, 2015, Personal Communication.
Also see CLF News Release.
A cure for hepatitis C now in reach for many Canadians!
March 24, 2015
Many provinces and territories now cover (or will soon) Harvoni and Sovaldi for the treatment of people with certain hepatitis C genotypes and treatment histories. PEI will also cover Holkira Pak. These highly effective and well-tolerated* medications are now accessible to many Canadians.
The provincial/territorial status for coverage for Harvoni (for the treatment of those with genotype 1) is as follows:
- British Columbia [see News Release]
- Manitoba (as of April 20, 2015) [see MB update]
- New Brunswick [see NB update]
- Ontario [see ON March 1, 2015 update]
- PEI (Holkira Pak: see PEI announcement; CATIE ]
- Quebec [search QC formulary for details]
- Yukon Territory [search YT formulary for details]
*Sovaldi and Holkira Pak may be used in combination with ribavirin or interferon in some cases (e.g., certain genotypes, treatment history). In these cases, side-effects may be more pronounced.
For more information, also see:
- Canadian Liver Foundation's press release
- Harvoni product monograph
- Sovaldi product monograph
- Holkira Pak product monograph
Sovaldi and Harvoni now available through BC PharmaCare!
March 24, 2015
As of today, BC PharmaCare will cover two new drugs for the treatment of hepatitis C: Harvoni (genotype 1) and Sovaldi (genotypes 1, 2 3).
Eligibility for coverage depends on meeting certain criteria, such as:
- No previous treatment for hepatitis C, or
- Failed treatment with older drugs
"This begins the path to eliminate hepatitis C in British Columbia", says Dr. Mel Krajden, medical head, hepatitis, BC Centre for Disease Control (source: BC Ministry of Health, News Release)
For more details see:
- BC Ministry of Health News Release
- Canadian Association for the Study of Liver treatment recommendations
- Harvoni product monograph
- Sovaldi product monograph
CASL has released new chronic HCV management recommendations!
February 6, 2015
The Canadian Association for the Study of the Liver (CASL) has released new guidelines for the management of chronic hepatitis C.Recommendations were published in the January/February 2015 volume of the Canadian Journal of Gastroenterology and Hepatology.
The guidelines include:
- Routine testing of patients with chronic hepatitis C (HCV)
- When peginterferon and ribavirin should not be used in treatment (contraindications)
- Treatment recommendations with newly approved antiviral agents, interferon-free alternatives, and interferon-containing alternatives for patients:
- with HCV genotypes 1 thru 6
- that are treatment naive or experienced, and
- whose liver is cirrhotic or noncirrhotic
Harvoni and HOLKIRA PAK approved by Health Canada
January 16, 2015
Health Canada recently approved Harvoni (ledipasvir + sofosbuvir) and HOLKIRA Pak (ombitasvir/paritaprevir/ritonavi/dasabuvir). Both are all-oral, short-course, interferon-free treatments for people over the age of 18 with the genotype 1 hepatitis C virus, including those with cirrhosis. Cure rates for both treatments are more than 90%.
Common side effects for both treatments (without ribavirin): Headache and Fatigue
Click here for more information on Harvoni
Click here for more information on HOLKIRA PAK.
Click here for health care provider treatment resources (University of Washington)
2014 Treatment News
BC PharmaCare approves SIMEPREVIR
Oct 31, 2014
BC Pharmacare approves SIMEPREVIR for the treatment of hepatitis C. For more details visit BC PharmaCare Formulary and type "Simeprevir" in the "Generic/brandname" box.
Health Canada approves Harvoni
Oct 16, 2014
Health Canada Issues Notice of Compliance for Harvoni™ (Ledispasvir/Sofosbuvir) for the Treatment of genotype 1 chronic hepatitis C.
European Medicines Agency approves Sovaldi
Sept 26, 2014
European Medicines Agency (EMA) approves Sovaldi for the treatment of chronic hepatitis C.
